La terapia nasal con flujo alto (nHFT) consiste en el suministro de gas respiratorio calentado y humidificado a través de una cánula nasal. Esto hace que el flujo de gas administrado sea superior al flujo respiratorio inspiratorio del paciente. En neonatología, un flujo superior a 1 l/min se considera una terapia con flujo alto, mientras que para los adultos, se deben aplicar valores proporcionalmente más elevados. (
Otros nombres de la terapia nHFT: terapia de cánulas nasales de flujo alto (HFNC) o terapia de cánulas nasales de flujo alto calentado y humidificado. Todos ellos designan la misma modalidad de terapia.
Colocación sencilla y cómoda
Complemento perfecto para el soporte respiratorio con nCPAP
Reducción del esfuerzo respiratorio
Disponible en los dispositivos medinCNOmini, medin-NC3 y medinSINDI
Si el flujo de gas respiratorio es superior al flujo inspiratorio del paciente, se puede reducir o evitar el suministro de aire ambiente a través de la nariz, a diferencia de lo que sucede en la terapia con cánulas nasales de flujo bajo convencional. Esto permite mejorar la oxigenación y la eficacia del suministro de oxígeno. (
El flujo de gas respiratorio expulsa y, por tanto, reduce el aire presente en el espacio muerto anatómico, lo que, a su vez, resulta en una disminución del esfuerzo respiratorio del paciente. El dióxido de carbono presente en la vía aérea superior al final de la espiración también se puede expulsar con un flujo de gas respiratorio alto. (
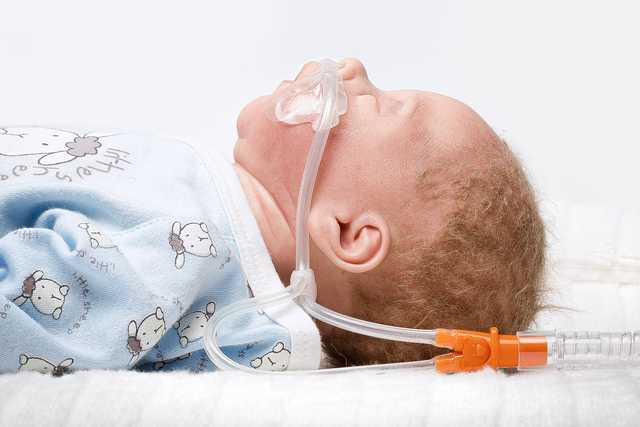
La colocación se lleva a cabo a través de nuestras cánulas nasales medinNuflow y es, por tanto, un proceso realmente sencillo y cómodo para el paciente. Para garantizar el efecto de lavado de la terapia, el tamaño de la cánula nasal se debe seleccionar teniendo en cuenta que al menos el 50 % de la abertura de la fosa nasal debe estar libre para dejar una vía de fuga intencionada. De este modo, el flujo de gas respiratorio podrá salir por la nariz a través de este espacio.
Durante la nHFT, no se puede medir la presión del paciente ni de los tubos. Para proteger al paciente de valores de presión no deseados, se debe mantener esta vía de fuga a modo de válvula de escape. (
medin ha integrado el modo nHFT en los dispositivos medinSINDI, medinCNOmini y medin-NC3.
Estos dispositivos permiten cambiar de forma sencilla e intuitiva de los modos nCPAP al modo nHFT sin necesidad de cambiar el sistema de tubos. Tan solo hace falta cambiar a la cánula nasal de medin en el generador de CPAP Medijet.
Además, se puede pausar la CPAP, utilizar el método madre canguro (contacto piel con piel) e implementar cambios en el tratamiento fácil y rápidamente. De este modo, los pacientes pueden recibir asistencia de manera flexible y personalizada.
Es necesario definir los siguientes parámetros: