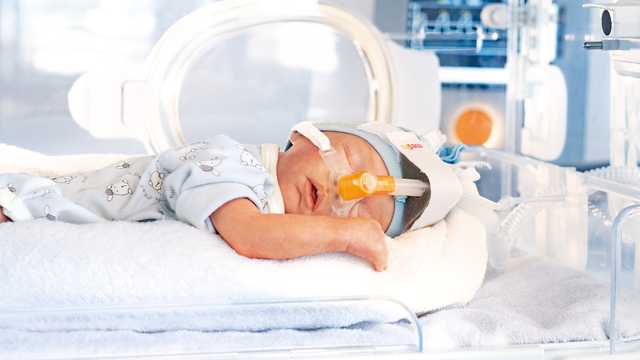
La apnea y las anomalías respiratorias afectan a prácticamente cualquier bebé prematuro o recién nacido. Cuanto más prematuro es el bebé, más acusada es la apnea. Este trastorno produce una interrupción de la mecánica respiratoria y el control de la respiración. La característica más importante de la apnea es el flujo de aire intermitente en las vías aéreas del paciente.
La apnea es clínicamente significativa para el paciente debido a los síntomas que la acompañan, como la bradicardia <80 c/min (disminución de la frecuencia cardíaca) y la hipoxia <80 % (disminución de la saturación de oxígeno). La finalidad de la profilaxis y el tratamiento de la apnea es evitar o minimizar sus efectos negativos (
Las respiraciones adicionales ayudan a estimular la respiración
Las vías aéreas se estabilizan y, como consecuencia, se facilita la respiración
Se detecta la respiración del propio paciente y el dispositivo cambia al modo CPAP
Disponible en los dispositivos medinCNO, medinCNOmini y medin-NC3
Los estudios han demostrado la eficacia de la CPAP nasal en este contexto y ya forma parte de las pautas aplicables para el tratamiento de la apnea y los síntomas que la acompañan (
Los mecanismos de acción son los siguientes:
El modo ApneaCPAP de medin se puede utilizar como tratamiento para la apnea, tanto para la profilaxis como para el tratamiento de apneas existentes.
El modo nCPAP detecta los episodios de apnea obstructiva y central gracias a la exclusiva tecnología MediTRIG y responde automáticamente suministrando respiraciones adicionales desde el dispositivo CPAP. El nivel de presión máxima de las respiraciones se ajusta con un segundo medidor de flujo electrónico y, a continuación, este ajuste se suma al flujo básico. Los parámetros de la función ApneaCPAP y del disparo MediTRIG se pueden adaptar a cada paciente de forma individual.
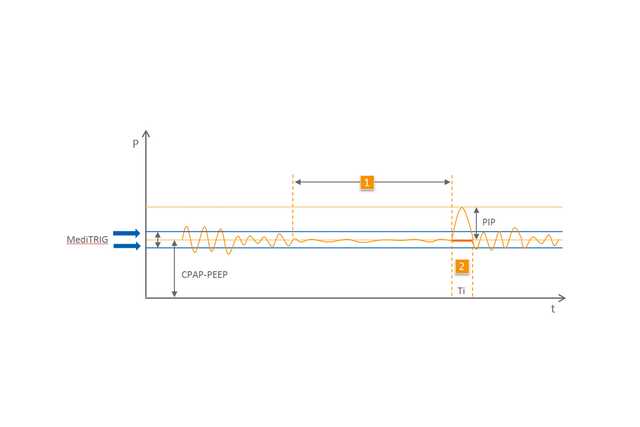
1 Apnea
2 Respiraciones automáticas